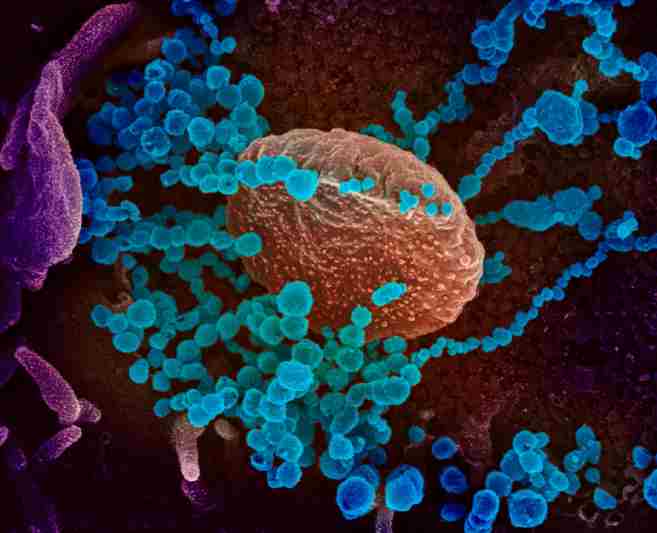
Contrary to previous theories, a new study reveals that the SARS-CoV-2 virus infects cells by binding to a single ACE2 receptor. Super-resolution microscopy revealed this, as well as a low density of ACE2 receptors on cell membranes, which raises the question of whether a virus particle could simultaneously bind to multiple receptors. The development of more effective COVID-19 treatment and prevention strategies may benefit from this new understanding.
The SARS-CoV-2 coronavirus pandemic that began in 2020 is now largely under control in Europe. However, the reason behind this virus’s rapid spread remains a mystery. In a paper published in the journal Angewandte Chemie, a group of researchers from the Julius Maximilian University of Würzburg (JMU) under the direction of Dr. Simone Backes, Dr. Gerti Beliu, and Prof. Dr. Markus Sauer demonstrated that a few of the previous hypotheses need to be rethought.
For instance, the virus does not simultaneously bind to multiple cell receptors with multiple surface proteins. An earlier attempt to explain how viruses increase their infectivity was based on this assumption. Additionally, the virus cannot dock with additional receptors after binding to a single receptor. Now that the Würzburg research group has demonstrated that a single virus binds to a single receptor, the possibility of a highly effective infection is open.
SARS-CoV-2 carries an average of 20-40 spike proteins on its surface, according to speculation. It does this by binding to ACE2 receptors within the membrane of its target cells, such as those in the human nose and throat. The cell cannot be infected after these receptors are blocked by antibodies. This recommends that the limiting of the infection to the ACE2 receptor is the definitive move toward contamination,” Sauer makes sense of.
Until now, it has not been possible to microscopically observe the ACE2 receptors and the interaction they have with the viral spike proteins. As a result, a lot was left up in the air, like whether the viruses bind to multiple receptors with multiple spikes to make it easier for them to enter the cell.
It was also thought that the receptors are in the membrane in groups of three or pairs so that they can bind to the trimeric spike proteins more effectively. or that only after binding to a spike protein do they form such groups. The density of ACE2 receptors in the membrane has a significant impact on both.
It was evident through super-resolution microscopy that the Würzburg researchers wanted to solve this mystery: They used dyes to label antibodies to make the receptors visible and easy to count. They accomplished this by employing the single-molecule sensitive super-resolution microscopy method dSTORM, which was developed by Markus Sauer’s group, as well as a variety of cell lines that serve as model systems for SARS-CoV infection.
It turned out that there are only one to two ACE2 receptors per square micrometer of cell membrane in Vero cells, which are frequently used as a model for SARS-CoV-2 infection. This is a small number: This number is frequently between 30 and 80 in other membrane receptors,” Sauer added.
“Approximately 500 nanometers separate neighboring ACE2 receptors on average.” According to Backes, it is therefore much larger than a virus particle, which is only 100 nanometers in size. The possibility that an infection molecule with various spike proteins can tie to different receptors at the same time is hence impossible, she adds.
The following is an unanswered question regarding ACE2 receptors: Are the receptors also found in the membrane in pairs or groups of three? No. They are very rare there. And it stays that way even after a viral spike protein binds to them, according to Beliu, the Rudolf Virchow Centre group leader. It is sufficient for an infection to have a single spike bind to a single receptor.
The JMU team was able to disprove many of the initial hypotheses regarding the viral particles’ interaction with multiple ACE2 receptors thanks to these findings. It also demonstrated, as expected, that host cells with higher ACE2 expression are more susceptible to infection. However, infection efficacy is also influenced by other factors, including the membrane’s lipid composition.
What follows?
The JMU group needs to accumulate however much definite information as could reasonably be expected about the cell passage system of Covids to all the more likely comprehend the disease cycle. In the end, this could help improve COVID-19 prevention and the development of better drugs. With high-resolution light sheet microscopy, the Würzburg researchers want to examine the entry mechanism as the next step.
An example: Drs. Patrick Eiring, Teresa Klein, Simone Backes, Marcel Streit, Marvin Jungblut, Sören Doose, Gerti Beliu, and Prof. Dr. Markus Sauer published “Coronaviruses Use ACE2 Monomers as Entry-Receptors” on March 27, 2023, in Angewandte Chemie.
The European Research Council, the German Research Foundation, and the German Federal Ministry of Education and Research all contributed funds to the described work.
Topics #ACE2 #COVID Theory #Microscopy #SARS-CoV-2