With a “Goldilocks” degree of reactivity—neither too much nor too little—this chemical element is so visually appealing that it was named after a goddess. This makes it a great option for use as a carbon cleansing tool.
The element is vanadium, and studies conducted by scientists at Oregon State University and published in Chemical Science have shown that molecules of vanadium peroxide can react and bind with carbon dioxide. This is a significant step toward developing more advanced methods for extracting carbon dioxide from the atmosphere.
The study is a component of a $24 million federal project to create new techniques for carbon dioxide capture through direct air, or DAC, a greenhouse gas linked to climate change that is produced when fossil fuels are burned.
While they’re still in their infancy, carbon-filtering facilities are starting to pop up all over the world. More advanced technologies are available for reducing carbon dioxide emissions at the source, like power plants. Scientists predict that both forms of carbon capture will probably be required if the planet is to escape the worst effects of climate change.
The Department of Energy has financed nine direct air capture projects, and May Nyman, an Oregon State chemistry professor in the College of Science, was selected to oversee one of them in 2021. Her group is studying the process by which some transition metal complexes react with air to extract carbon dioxide and transform it into a metal carbonate, which is akin to the carbonate found in a variety of naturally occurring minerals.
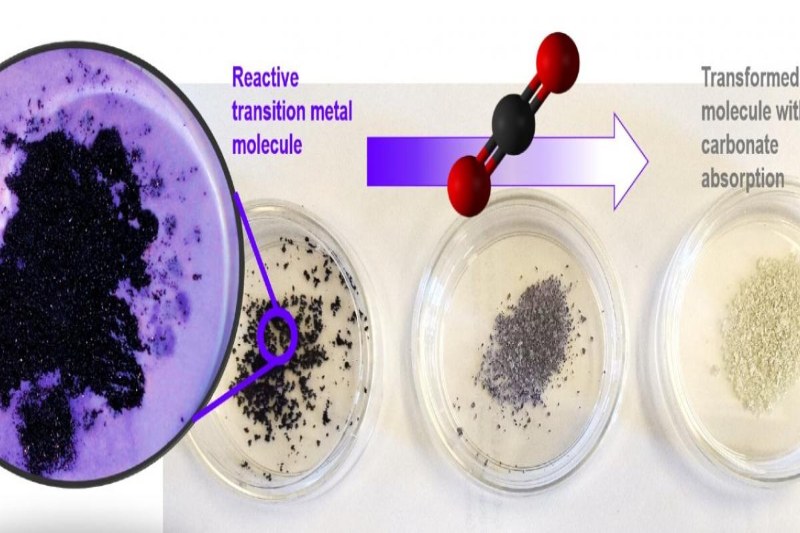
The shift of electrons from low energy to high energy states and back again, giving rise to different colors, is the reason behind the moniker “transition metals,” which are found close to the center of the periodic table. The Scandinavian goddess of love, Vanadis, was named for her in old Norse mythology and is claimed to have been so lovely that her tears changed to gold. This led scientists to choose vanadium for their study.
According to Nyman, the density of carbon dioxide in the atmosphere is 400 parts per million. This indicates that 400 air molecules, or 0.04%, are carbon dioxide for every million air molecules.
“A challenge with direct air capture is finding molecules or materials that are selective enough, or other reactions with more abundant air molecules, such as reactions with water, will outcompete the reaction with CO2.””Our team synthesized a series of molecules that contain three parts that are important in removing carbon dioxide from the atmosphere, and they work together.”
One component was vanadium, which gets its name from the variety of exquisite colors it can display. The other component was peroxide, which joined with the vanadium to form a bond. According to Nyman, a vanadium peroxide molecule requires alkali cations for charge balancing because it is negatively charged. For this investigation, the researchers employed potassium, rubidium, and cesium alkali cations.
She continued by saying that the partners also experimented with replacing vanadium with other metals from the same neighborhood on the periodic table.
“Tungsten, niobium and tantalum were not as effective in this chemical form,” Nyman said. “On the other hand, molybdenum was so reactive it exploded sometimes.”
Furthermore, the researchers replaced the alkalis with ammonium and tetramethyl ammonium, the former of which has a mild acidity. The chemicals in question exhibited no reaction at all, a mystery that the researchers are still attempting to solve.
“And when we removed the peroxide, again, not so much reactivity,” Nyman explained. “In this sense, vanadium peroxide is a beautiful, purple Goldilocks that becomes golden when exposed to air and binds a carbon dioxide molecule.”
She points out that one additional important property of vanadium is that it permits the captured carbon dioxide to be released at a very moderate temperature—roughly 200°C.
“That’s compared to almost 700°C when it is bonded to potassium, lithium or sodium, other metals used for carbon capture,” she said. “Being able to rerelease the captured CO2 enables reuse of the carbon capture materials, and the lower the temperature required for doing that, the less energy that’s needed and the smaller the cost. There are some very clever ideas about reuse of captured carbon already being implemented—for example, piping the captured CO2 into a greenhouse to grow plants.”
Tim Zuehlsdorff, an assistant professor of theoretical and physical chemistry at Oregon State, and Eduard Garrido, a postdoctoral researcher, were among the other authors of the work.
“I’m also really proud of the hard work of the graduate students in my lab, Zhiwei Mao and Karlie Bach, and undergraduate Taylor Linsday,” Nyman said. “This is a brand new area for my lab, as well as for Tim Zuehlsdorff, who supervised Ph.D. student Jacob Hirschi on the computational studies to explain the reaction mechanisms. Starting a new area of study involves many unknowns.”
Topics #Carbon Capture #Vanadium Research