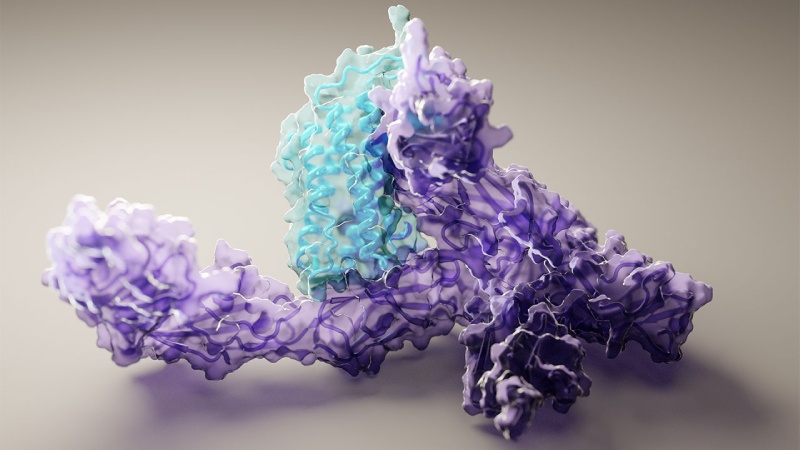
Proteins are involved in the majority of inside of cells that keep us mammals alive, breathing, and thinking. They facilitate internal communication, control the fundamental metabolic processes of a cell, and help in the transformation of DNA information into more proteins. And the capacity of the protein’s chain of amino acids to fold into a complex yet precise three-dimensional form that allows it to operate is the basis for all of that.
Understanding that 3D shape up until this decade required separating the protein and putting it through a labor- and time-intensive process to figure out its structure. However, this was changed by the efforts of DeepMind, a Google AI division that produced Alpha Fold in 2021, as well as a related academic project that came shortly after. Not all proteins could be solved with high confidence by the software, and it had difficulty with higher proteins. However, a large number of its forecasts proved to be amazingly accurate.
Still, these frameworks communicated only part of the story. Almost all proteins need to interact with other proteins, DNA, chemicals, membranes, and other things in order to function. In addition, only a few protein-protein interactions could be addressed by AlphaFold’s first iteration; the others were left as mysteries. With major changes made to or a complete replacement of its underlying engine, AlphaFold version 3 is now available, as announced by DeepMind. The program can now handle a number of extra protein modifications and interactions as a result of these changes.
The initial version of AlphaFold was dependent on two fundamental software features. One of those considered the limitations of evolution on a protein. By examining the same protein across species, you may determine which regions are consistently the same and, thus, most likely essential to the function of the protein. Because of their centrality, they are likely to constantly be oriented and located in the same places within the protein’s structure. In order to accomplish this, the first AlphaFold gathered as many protein variants as it could and aligned their sequences to identify the areas that had little variation.
However, doing so is computationally expensive because there are more limitations to overcome the more proteins you line up. The AlphaFold team continued to identify several related proteins in the updated version, but they mostly used combinations of protein sequences from the set of related ones for their alignments. While it’s much more computationally efficient, this is probably not as information-rich as a multi-alignment, and the information that’s lost doesn’t seem to be essential for determining protein structures.
A separate software module used these alignments to determine the spatial correlations between amino acid pairs in the target protein. Then, using code that considered certain of the physical features of amino acids, such as which parts of an amino acid might spin in respect to one another, etc., those relationships were translated into spatial coordinates for each atom.
Atomic position prediction in AlphaFold 3 is done via a diffusion module that is trained with both known structures and variants of the structure that have noise (atom locations being shifted) added to them. This makes it possible for the diffusion module to take the approximate locations given by relative positions and turn them into precise estimates of each atom’s location inside the protein. It may determine the typical functions of amino acids from enough structural information, so it doesn’t need to be informed about their physical features.
To make the diffusion module function, DeepMind needed to train on two distinct noise levels: one where the noise involved shifting the large-scale structure of the protein, which in turn affected the location of many atoms, and another where the noise involved shifting atom locations while maintaining the overall structure.
The researchers discovered that AlphaFold 3 needed to see approximately 20,000 protein structure examples in order to correctly identify roughly 97% of a test set’s structures. It began to correctly detect protein-protein interactions at that frequency by the 60,000th instance. Importantly, it also began to correctly assemble proteins into compounds with other molecules.
Topics #DeepMind #diffusion engine #DNA #news #protein #protein folding #software